New coronavirus cases exceeded 40,000 for a second consecutive day on Thursday as the omicron-fueled fourth wave continues to gather strength.
A day after a pandemic high of over 44,000 confirmed cases was reported, new infections numbered 43,523 on Thursday.
Mexico’s accumulated case tally rose to almost 4.26 million, while estimated active cases hit a new high of over 257,000. The official COVID-19 death toll increased by 148 to 300,912.
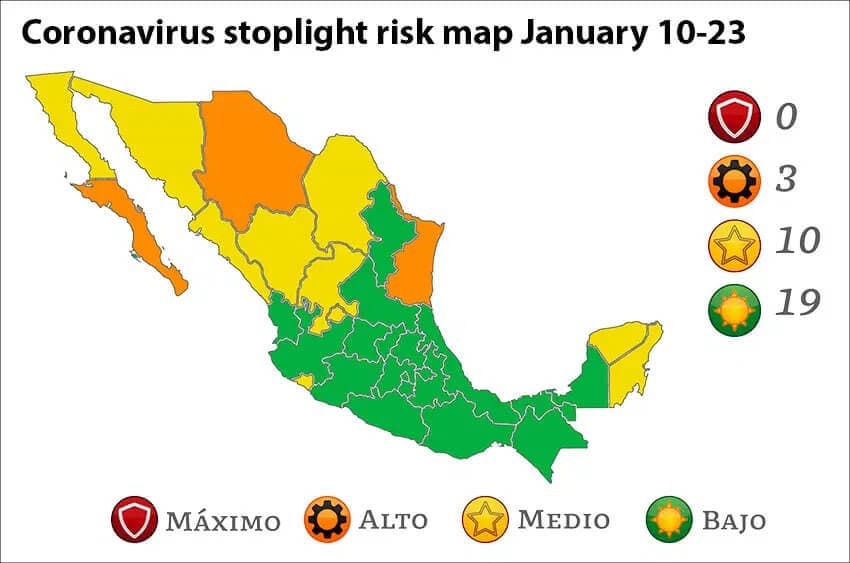
Baja California Sur remains the country’s COVID epicenter with more than 1,000 active cases per 100,000 people. Mexico City, which has around 60,000 active infections, ranks second with over 600 per 100,000 residents.
Twelve other states have between 200 and 400 active cases per 100,000 people. They are San Luis Potosí, Quintana Roo, Zacatecas, Colima, Yucatán, Tabasco, Nayarit, Coahuila, Nuevo León, Baja California, Querétaro and Durango.
In other COVID-19 news:
• The national occupancy rate for general care beds in COVID wards increased by two points to 26%, the federal Health Ministry said Thursday. The occupancy rate for beds with ventilators rose one point to 16%.
• Almost 938,000 vaccine doses were administered Thursday, lifting the total number of shots given to over 154.6 million. About two-thirds of Mexicans have received at least one vaccine dose, while around 60% are fully vaccinated. More than 80% of adults are vaccinated.
• The Health Ministry is now recommending that people suspected to have COVID-19 isolate for seven rather than 14 days.
Infectious disease specialist Alejandro Macías said on Twitter that the omicron variant “enters and leaves quickly,” meaning that infected people will generally develop symptoms shortly after exposure and get better soon.
He said that a return to work after an isolation period of seven days is “reasonable.” With a 14-day isolation period, “we will be left without people to work,” he tweeted Thursday.
• Health regulator Cofepris has granted emergency use authorization to Paxlovid, the anti-viral COVID pill produced by the United States pharmaceutical company Pfizer.
Cofepris said in a statement Friday that it is the first health regulator in Latin America to approve the drug. It also said that treatment with the medication can reduce death and the need for hospitalization by up to 88%.
Mexico News Daily